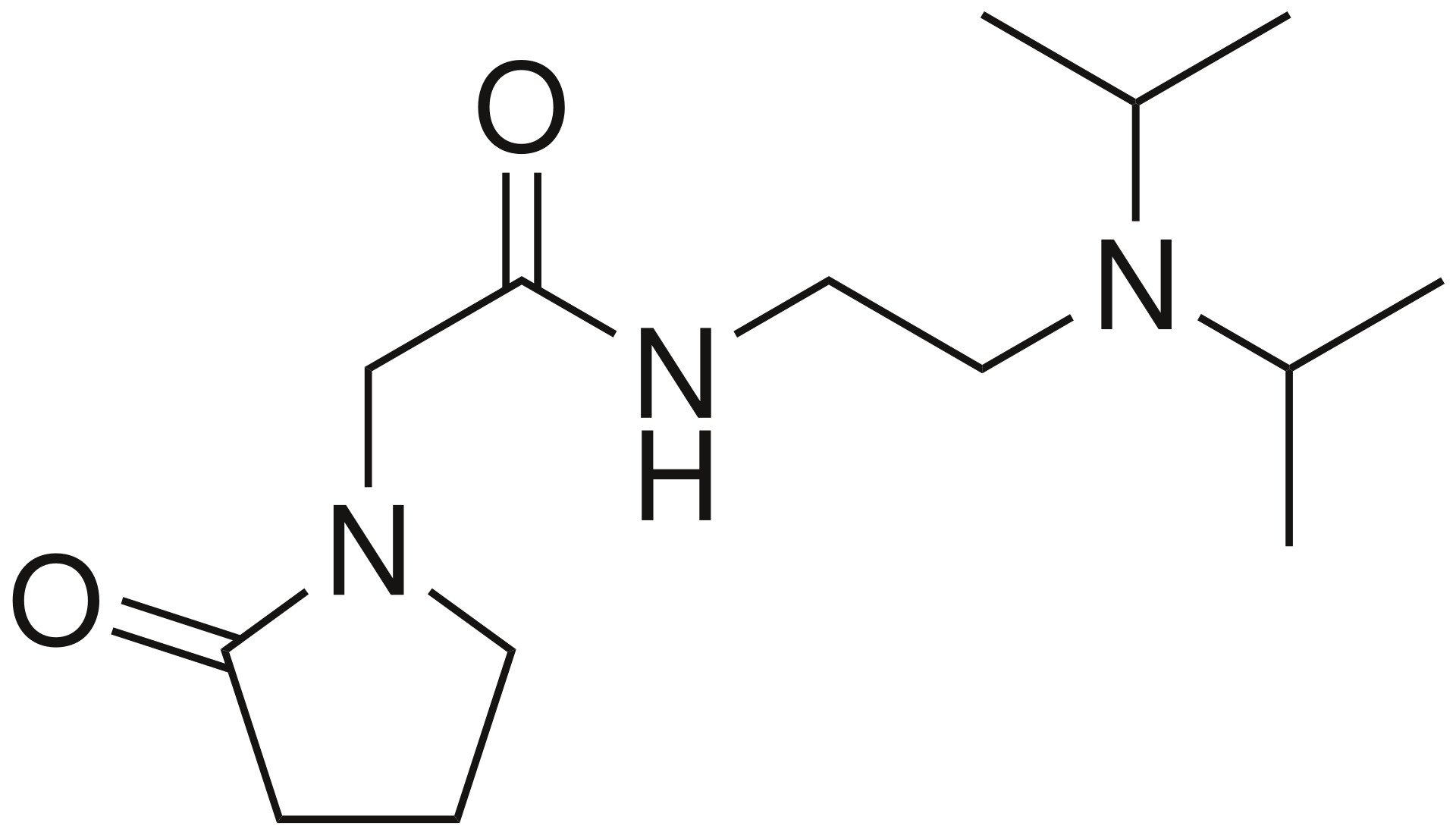
Pramiracetam
N-[2-(diisopropylamino)ethyl]-2-oxo-1-pyrrolidineacetamide
A lipophilic racetam nootropic with high potency and exceptional cholinergic modulation. Known for significant memory enhancement, increased mental endurance, and pronounced effects on information processing and recall in both clinical and research settings.
Available as:
Formula
C₁₄H₂₇N₃O₂
Category
Racetam
Half-Life
4.5-7.5 hours
Standard Dose
300-1200 mg
Chemical Profile
Mechanisms of Action
Pramiracetam exhibits unique neuropharmacological effects through several distinct neurochemical pathways, with particularly strong actions on cholinergic systems:
High-Affinity Choline Uptake (HACU)
Significantly increases high-affinity choline uptake in the hippocampus, enhancing acetylcholine synthesis and release. This mechanism is 2-3 times more potent than piracetam and is directly linked to improved memory formation and cognitive processing.
Prefrontal Cortex Activation
Exhibits pronounced activity in the prefrontal cortex, enhancing executive functions including working memory, attention, and decision-making processes. This contributes to its distinctive effects on logical thinking and information management.
Membrane Fluidity Modulation
Alters neuronal membrane fluidity through its lipophilic properties, enhancing signal transduction efficiency and neurotransmitter receptor function. This mechanism facilitates improved synaptic transmission and neuroplasticity.
Nitric Oxide Regulation
Modulates nitric oxide (NO) production in cerebral tissues, potentially enhancing cerebral blood flow and microcirculation. This improves oxygen and glucose delivery to neurons, supporting cognitive endurance and sustained mental performance.
Neuroprotection
Demonstrates significant neuroprotective properties, particularly against scopolamine-induced amnesia and oxidative stress. These effects are mediated through antioxidant mechanisms and stabilization of cell membrane structures.
Unique Pharmacokinetic Profile
Exhibits high lipid solubility and effective blood-brain barrier penetration, allowing for rapid onset of action and efficient distribution to neural tissues. This contributes to the compound's high potency and relatively long duration of effects.
Note: Pramiracetam is estimated to be 15-30 times more potent than piracetam in enhancing cognitive function, with a significantly higher affinity for cholinergic mechanisms. Its effects are predominantly on memory formation and information processing rather than mood or anxiety modulation.
Clinical Applications & Efficacy
Neurodegenerative & Vascular Dementias
Menarini conducted clinical trials in Italy and Eastern Europe evaluating pramiracetam's efficacy in treating memory and attention deficits in aging patients with neurodegenerative and vascular dementias. These studies formed the basis for pramiracetam's approval under the brand name Pramistar in these regions.
Warner-Lambert also conducted Phase II clinical trials for Alzheimer's Disease, though results were mixed. Despite this, pramiracetam continues to be prescribed for various forms of dementia in several European countries, where clinical experience suggests benefits for memory function and cognitive processing.
Traumatic Brain Injury & Cognitive Recovery
Cambridge Neuroscience, Inc. (CNI) conducted a clinical trial in four individuals experiencing cognitive problems following traumatic head injury. Although the sample size was small, the results were promising enough to warrant further investigation into pramiracetam's potential for enhancing cognitive recovery after brain trauma.
Additional small-scale trials conducted in Ukraine examined pramiracetam's effects in patients with concussion, providing preliminary evidence for its application in post-traumatic cognitive rehabilitation. These studies suggest pramiracetam may accelerate cognitive recovery and improve functional outcomes following brain injury.
Scopolamine-Induced Amnesia
A notable study conducted in Italy investigated pramiracetam's ability to counteract scopolamine-induced amnesia in healthy subjects. Scopolamine, an anticholinergic compound, is used experimentally to model memory impairment similar to that seen in Alzheimer's disease.
The research demonstrated that pramiracetam significantly attenuated the memory-impairing effects of scopolamine, providing strong evidence for its cholinergic mechanisms and potential applications in conditions characterized by cholinergic dysfunction, such as various forms of dementia and age-associated memory impairment.
Cerebrovascular Disease
Clinical investigations in Ukraine examined pramiracetam's efficacy in patients with cerebrovascular disease. These studies evaluated cognitive function, memory performance, and quality of life metrics in patients with vascular cognitive impairment not reaching the threshold of dementia.
Results indicated improvements in several cognitive domains, particularly in information processing speed, attention, and memory consolidation. These findings support pramiracetam's potential role in managing cognitive manifestations of cerebrovascular disease and possibly mitigating cognitive decline in at-risk populations.
Adjunct in Major Depressive Disorder
Warner-Lambert began developing pramiracetam as an orphan drug to be used as an adjunct to electroconvulsive therapy (ECT) for major depressive disorder. This application was pursued in part to leverage the administrative exclusivity provided by orphan drug status in the United States.
The rationale was based on pramiracetam's potential to mitigate the cognitive side effects of ECT while possibly enhancing treatment efficacy through its neurotrophic and neuroprotective mechanisms. This development pathway was later discontinued, with the orphan designation eventually withdrawn.
FDA Orphan Drug Designations and Approvals Database. Cambridge Neuroscience Developing Warner-Lambert's Pramiracetam (1991).
Pharmacokinetic Considerations
Pramiracetam exhibits distinct pharmacokinetic properties due to its lipophilic nature. Oral bioavailability is estimated at 95%, with peak plasma concentrations reached within 30 minutes to 1 hour. The compound has a half-life of approximately 4.5-7.5 hours, allowing for twice-daily dosing. Pramiracetam crosses the blood-brain barrier efficiently, with brain concentrations significantly higher than many other racetams. Unlike water-soluble racetams, pramiracetam undergoes both hepatic metabolism and renal excretion, with approximately 60% excreted unchanged in urine and 40% metabolized by the liver.
Side Effects & Considerations
Pramiracetam generally displays a favorable safety profile with relatively few adverse effects in most users:
Neurological/Psychological
- •Headache (typically mild and transient)
- •Occasional insomnia if taken late in day
- •Increased mental fatigue after effects wear off
- •Rare reports of irritability
Gastrointestinal
- •Burning sensation in throat (due to lipophilic nature)
- •Occasional stomach discomfort
- •Nausea (usually if taken without food)
Special Considerations
- •Best taken with fatty meals to enhance absorption
- •Potential for acetylcholine depletion with prolonged use
- •May interact with other cholinergic compounds
Tolerability Profile: Clinical studies have demonstrated pramiracetam's favorable tolerability even at higher dosages. Its side effect profile is generally milder than many conventional cognitive enhancers, with no serious adverse events reported in clinical trials. Unlike some stimulants or cholinesterase inhibitors, pramiracetam typically does not cause significant cardiovascular effects or severe gastrointestinal disturbances.
Regulatory & Legal Status
United States
- •Not FDA approved for medical use
- •Previously had orphan drug designation (withdrawn)
- •Unscheduled compound
- •Available primarily as research compound
International Status
- •Italy: Approved and marketed as Pramistar
- •Eastern Europe: Available by prescription in several countries
- •Russia: Approved for cognitive disorders
- •Australia: Schedule 4 (prescription only) substance
- •Asia: Regulatory status varies by country
Historical Context
Pramiracetam was discovered by scientists at Parke-Davis (a division of Warner-Lambert) in the late 1970s as part of their research into more potent derivatives of piracetam. It was designed to enhance the cognitive effects of the original racetam while improving lipophilicity and blood-brain barrier penetration.
"Pramiracetam represents a significant advancement in racetam pharmacology, incorporating diisopropylamine substitutions that dramatically enhance potency and cholinergic activity compared to first-generation compounds in this class."— Journal of Medicinal Chemistry, 1979
Patents for pramiracetam expired in 1996, after which Warner-Lambert pursued various clinical applications including Alzheimer's disease treatment. Following mixed results in Phase II trials, the company shifted focus to developing it as an orphan drug for use as an adjunct to electroconvulsive therapy for major depressive disorder.
Warner-Lambert licensed European rights to Menarini, which continued developing pramiracetam for dementia treatments. In 1991, they licensed US and other non-European rights to Cambridge Neuroscience, Inc. (CNI), which pursued indications for cognitive recovery after stroke or traumatic brain injury, as well as the ECT adjunct indication.
Today, pramiracetam remains available as a prescription medication in parts of Europe and is used primarily for age-related cognitive decline, neurodegenerative conditions, and cognitive rehabilitation following brain injury.

Premium Pramiracetam
Enhance your cognitive performance with science-backed, high-quality nootropics.
Benefits
- One of the most potent racetams for long-term memory
- Significant enhancement of high-level cognitive processes
- Long half-life allowing for once or twice daily dosing
- Uniquely high affinity for choline uptake sites
Considerations
- Lipid-soluble - take with fats for best absorption
- Distinctly bitter taste when taken sublingually
- Higher cost than many other racetams
- May reduce emotional reactivity during peak effects
Free shipping on orders above $50
Scientific References
This information is provided for educational purposes only and is not intended as medical advice. Consult a healthcare professional before using any nootropic compound.
Content based on peer-reviewed research, clinical studies, and pharmacological databases. Last updated: 4/12/2025