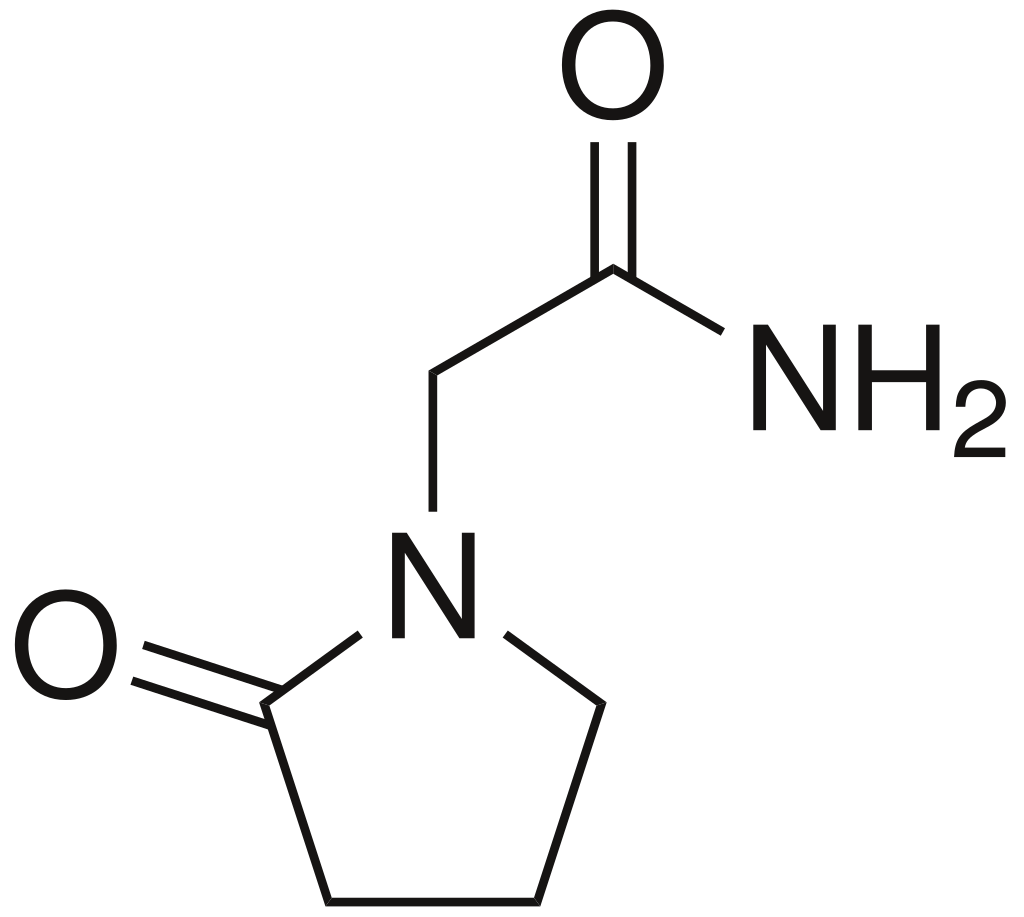
Piracetam
2-oxo-1-pyrrolidine acetamide
The original racetam nootropic discovered in 1964, known for enhancing memory and cognitive function. First synthesized by Dr. Corneliu E. Giurgea in 1964, it established the entire nootropic classification.
Available as:
Formula
C6H10N2O2
Category
Racetam
Discovered
1964
Standard Dose
800-2400 mg
Chemical Profile
Mechanisms of Action
Piracetam exhibits multiple neurophysiological effects through various mechanisms:
Membrane Fluidity Modulation
Enhances neuronal membrane fluidity, increasing oxygen utilization and glucose metabolism in hypoxic conditions.
Neurotransmitter Modulation
Acts as a positive allosteric modulator of AMPA receptors. Enhances acetylcholine transmission via muscarinic cholinergic receptors.
Ion Channel Interaction
Inhibits N-type calcium channels (IC50 of 3 μM in rat neurons) and may modulate Na+ and K+ channels, increasing neuronal excitability.
Mitochondrial Function
Increases cytochrome b5 synthesis and enhances permeability of Krebs cycle intermediates through mitochondrial membranes.
Microcirculation Enhancement
Reduces erythrocyte adhesion to vascular endothelium, diminishing vasospasm severity and improving cerebral microcirculation.
Metabolic Enhancement
Increases ATP metabolism and adenylate kinase activity in neurons.
Note: Piracetam does not affect GABA metabolism or GABA receptors despite its structural similarity to GABA.
Clinical Applications & Efficacy
Cognitive Disorders & Dementia
Winblad's comprehensive 2005 review analyzed multiple clinical trials of piracetam in elderly patients with cognitive impairment, finding significant improvements in global impression of change, cognitive performance measures, and clinical improvement ratings compared to placebo. The research demonstrated particular efficacy in patients with age-related cognitive decline rather than established dementia.
However, the Cochrane Database of Systematic Reviews (Flicker & Grimley Evans, 2001) found insufficient evidence to support piracetam as a dementia treatment, citing methodological limitations in available studies. This analysis emphasized the need for more rigorous randomized controlled trials with standardized outcome measures to definitively establish piracetam's efficacy in neurodegenerative conditions.
Myoclonus & Neurological Disorders
The strongest clinical evidence for piracetam exists in the treatment of cortical myoclonus. Malykh and Sadaie's 2010 review documented significant improvement in myoclonus scores and functional disability in patients with progressive myoclonic epilepsy and post-hypoxic myoclonus, often at higher dosages (7.2-24g daily) than used in cognitive enhancement applications.
Based on this evidence, piracetam (as Nootropil) received regulatory approval in the United Kingdom specifically for myoclonus treatment, with the electronic Medicines Compendium (eMC) recommending its use for cortical myoclonus either as monotherapy or adjunctive treatment. Long-term follow-up studies have demonstrated sustained benefits with minimal tolerance development.
Stroke & Ischemic Conditions
A comprehensive 2012 Cochrane Review by Ricci et al. analyzed data from 11 randomized controlled trials (n=1,983 patients), concluding that piracetam showed no statistically significant benefit when administered within the first 48 hours of acute ischemic stroke onset. The authors found insufficient evidence to support routine clinical use in this context.
However, more promising results emerged for post-stroke aphasia rehabilitation. Zhang et al. (2016) conducted a systematic review and meta-analysis of randomized controlled trials, finding that piracetam significantly improved language recovery compared to conventional rehabilitation alone. Their analysis revealed particular benefits for comprehension, repetition, reading, and naming abilities when piracetam was incorporated into standard speech therapy protocols.
Sickle Cell Disease & Hematological Applications
Al Hajeri and Fedorowicz (2016) conducted a Cochrane systematic review of randomized controlled trials evaluating piracetam for reducing painful crises in sickle cell disease. Their analysis of three studies (n=169 participants) found limited evidence suggesting that piracetam may reduce the number of painful days and hospital admissions compared to placebo, though methodological limitations were noted.
The mechanism appears to involve piracetam's effects on erythrocyte deformability and microcirculation, as detailed by Winnicka et al. (2005). Their research demonstrated that piracetam reduces erythrocyte membrane rigidity and increases cell deformability, potentially improving blood flow in microvascular conditions including sickle cell anemia and Raynaud's phenomenon.
ADHD & Cognitive Enhancement
Limited clinical evidence exists for piracetam as a monotherapy in attention deficit hyperactivity disorder (ADHD). However, Zavadenko and Suvorinova (2008) conducted a controlled trial examining piracetam as an adjunctive therapy alongside atomoxetine in children with ADHD. Their findings showed improved treatment outcomes with the combination therapy compared to atomoxetine alone.
The cognitive enhancement effects in healthy individuals remain controversial. A 2008 report by the British Academy of Medical Sciences noted substantial methodological limitations in studies examining nootropic effects of piracetam in non-impaired individuals. The report highlighted the need for more rigorous randomized controlled trials with appropriate cognitive endpoints before conclusions about enhancement effects could be drawn.
Pharmacokinetic Considerations
Piracetam exhibits nearly complete oral bioavailability (100%) with minimal protein binding and metabolism. Studies by Yeh et al. (2006) established that piracetam readily crosses the blood-brain barrier, with CSF concentrations reaching approximately 70-90% of plasma levels. Tacconi & Wurtman (1986) demonstrated that piracetam is primarily excreted unchanged in urine, with an elimination half-life of 4-5 hours in healthy adults, potentially extending to 8-12 hours in elderly patients with reduced renal function. This pharmacokinetic profile allows for consistent dosing schedules, typically 2-3 times daily administration to maintain therapeutic concentrations.
Side Effects & Considerations
Piracetam exhibits an extremely favorable safety profile with minimal adverse effects at therapeutic dosages. Reported side effects include:
Neurological/Psychological
- •Headache
- •Nervousness
- •Gastrointestinal discomfort
- •Insomnia
- •Hyperkinesia
- •Depression (rare)
Physical
- •Weight gain
- •Weakness
- •Somnolence
Contraindications
- •Cerebral hemorrhage
- •Severe renal impairment
Regulatory & Legal Status
United States
- •Not FDA approved for medical use
- •Legal to possess without prescription
- •Cannot be sold as dietary supplement or drug
- •Available as research compound
International Status
- •United Kingdom: Prescription medication for myoclonus (Nootropil)
- •Japan: Approved prescription medication
- •Czech Republic: Available without prescription
- •Hungary: OTC as Memoril Mite (600mg)
- •Canada: No DIN, but legal for personal import
- •European Union: Available as Nootropil, Lucetam, Breinox
Historical Context
Synthesized between the late 1950s and 1964 by Dr. Corneliu E. Giurgea at UCB Laboratories in Belgium, Piracetam was initially investigated for epilepsy treatment. Dr. Giurgea coined the term "nootropic" specifically to describe Piracetam's unique cognitive-enhancing properties without sedative or stimulant effects.
"Nootropic: from the Greek 'noos' (mind) and 'tropein' (towards) - compounds that act towards the mind."— Dr. Corneliu E. Giurgea, 1972
The compound has maintained continuous research interest for over five decades, with extensive clinical investigation across multiple neurological conditions. In 2009, it gained popularity among students seeking cognitive enhancement, establishing its position in the modern nootropics movement.
As the prototypical racetam, Piracetam's molecular structure has served as the foundation for developing numerous related compounds including Aniracetam, Oxiracetam, Pramiracetam, and the anticonvulsant medications Levetiracetam (Keppra) and Brivaracetam (Briviact).

Premium Piracetam
Enhance your cognitive performance with science-backed, high-quality nootropics.
Benefits
- The original and most extensively researched racetam
- Enhances membrane fluidity and cellular metabolism
- Neuroprotective effects with minimal side effects
- Improves cognitive function in healthy and impaired individuals
Considerations
- Higher doses required compared to newer racetams
- Effects build gradually over consistent use
- May cause headaches if choline is deficient
- Water-soluble with good safety profile
Free shipping on orders above $50
Scientific References
This information is provided for educational purposes only and is not intended as medical advice. Consult a healthcare professional before using any nootropic compound.
Content based on peer-reviewed research, clinical studies, and pharmacological databases. Last updated: 4/12/2025