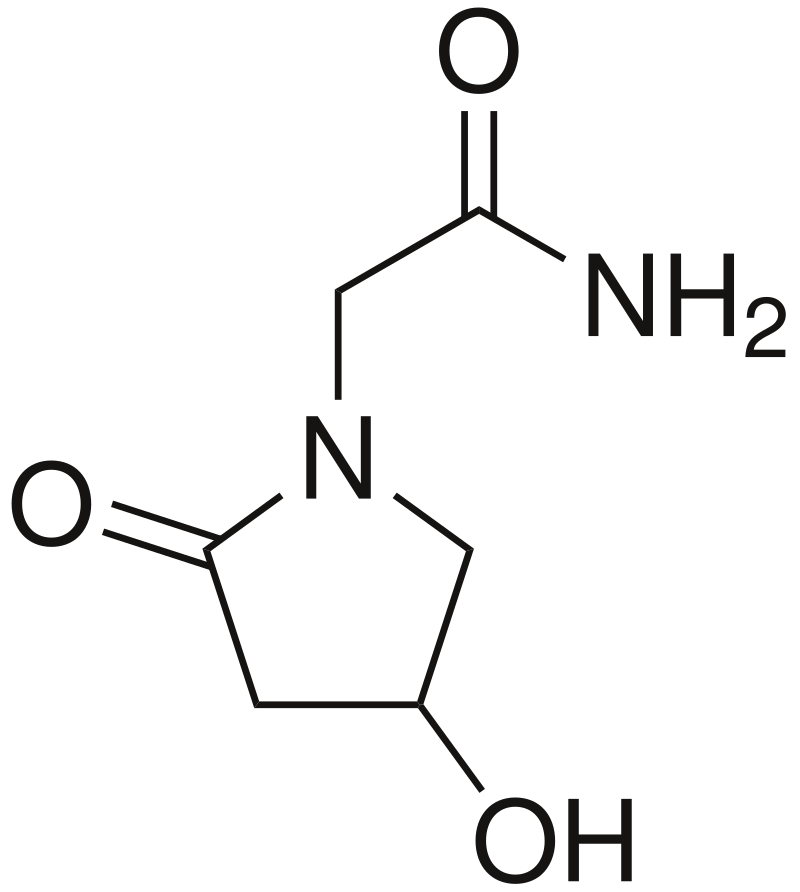
Oxiracetam
4-Hydroxy-2-oxopyrrolidin-1-yl)acetamide
A water-soluble racetam nootropic with cognitive enhancement properties and potent modulation of cholinergic neurotransmission. Known for improved memory formation, logical thinking, and mild stimulatory effects in both clinical and research settings.
Available as:
Formula
C₆H₁₀N₂O₃
Category
Racetam
Half-Life
8 hours
Standard Dose
800-1600 mg
Chemical Profile
Mechanisms of Action
Oxiracetam exhibits multiple neuropharmacological effects through several distinct neurochemical pathways:
Cholinergic System Enhancement
Increases acetylcholine release and potentiates cholinergic neurotransmission in the hippocampus and cortex, improving synaptic efficiency and memory formation processes.
Protein Kinase C Activation
Increases membrane-bound protein kinase C (PKC) activity, particularly in the hippocampus, which correlates with improved spatial learning performance and memory consolidation.
Glutamatergic Modulation
Enhances glutamatergic neurotransmission via AMPA receptor modulation, contributing to synaptic plasticity and long-term potentiation necessary for learning.
Metabolic Enhancement
Increases glucose utilization and cerebral metabolism in the brain, particularly in regions associated with cognitive processing, improving overall neural energy efficiency.
Neuroplasticity Promotion
Enhances long-term potentiation (LTP) and stimulates neurite outgrowth, facilitating brain adaptation and recovery following injury or neurodegeneration.
Neuroprotection
Exhibits protective effects against hypoxia and cerebral hypoperfusion, potentially through antioxidant mechanisms and reduction of excitotoxicity.
Note: Oxiracetam exhibits a significant concentration gradient between blood and brain tissues, with brain concentrations reaching approximately 5.3% of blood concentrations. The highest concentrations are found in the septum pellucidum and hippocampus.
Clinical Applications & Efficacy
Cognitive Disorders & Dementia
Multiple clinical studies have investigated oxiracetam's effects on dementia patients. Parnetti et al. (1989) conducted a long-term therapy study comparing oxiracetam to placebo in patients with Alzheimer's and multi-infarct dementia, finding significant improvements in memory, attention, and spatial orientation in the oxiracetam group.
A double-blind, placebo-controlled study by Bottini et al. (1992) further supported these findings, noting improved cognitive performance in patients with mild to moderate dementia, while Villardita (1992) observed consistent cognitive benefits across various dementia subtypes when oxiracetam was administered at 1600 mg daily.
Cognitive Enhancement & Learning Improvement
Fordyce et al. (1995) demonstrated that oxiracetam significantly enhances hippocampally-mediated learning and increases protein kinase C activity in learning-impaired mice. Their study showed improved performance in the Morris water navigation task, establishing a molecular mechanism for oxiracetam's cognitive enhancement effects.
Comparative research by Mondadori (1986) evaluated oxiracetam against piracetam in various animal models of learning and memory, finding oxiracetam to be more potent in several experimental paradigms, particularly in tasks requiring complex cognitive processing and retention of learned information.
Neuroprotection in Special Conditions
Recent research by Hu et al. (2017) investigated oxiracetam's protective effects against cognitive impairment at high altitude. Their findings revealed that oxiracetam administration significantly reduced cognitive deficits associated with hypoxic conditions, suggesting potential applications for individuals experiencing cognitive impairment due to reduced oxygen availability.
Studies examining oxiracetam's effects in post-concussion syndrome and organic brain syndromes have shown encouraging results. Itil et al. (1986) documented improved cognitive function in patients with these conditions, with benefits observed in attention, concentration, and information processing speed.
Stereoisomer-Specific Effects
Li et al. (2017) conducted groundbreaking research showing that the (S)-oxiracetam enantiomer is the active ingredient responsible for alleviating cognitive impairment induced by chronic cerebral hypoperfusion in rats. This study demonstrated that the therapeutic effects of oxiracetam are stereospecific, with the (S)-enantiomer providing superior cognitive enhancement compared to the (R)-enantiomer.
This research has important implications for future development of more potent and selective oxiracetam derivatives, suggesting that isolated (S)-oxiracetam could potentially offer enhanced therapeutic efficacy for cognitive disorders with lower required dosages.
Comparative Efficacy
Krylova et al. (1991) conducted a direct comparison between oxiracetam and piracetam, finding that oxiracetam demonstrated superior nootropic properties across multiple cognitive domains. Their analysis showed oxiracetam was particularly effective at improving memory consolidation and recall, with a more favorable potency profile compared to the parent compound piracetam.
This comparative advantage was further supported by Gouliaev and Senning's (1994) comprehensive review of racetam nootropics, which positioned oxiracetam as having enhanced cognitive effects due to its hydroxylated structure, allowing for increased blood-brain barrier penetration and improved receptor interactions compared to piracetam.
Pharmacokinetic Considerations
Oxiracetam exhibits favorable pharmacokinetic properties with oral bioavailability ranging from 56-82%. Peak plasma concentrations are reached within 1-3 hours after oral administration, with maximal serum concentrations of 19-31 μg/ml following 800-2000 mg doses. The compound has a typical half-life of 8 hours in healthy individuals, extending to 10-68 hours in those with renal impairment. Approximately 84% is excreted unchanged in urine, indicating minimal hepatic metabolism. Clearance rates range from 9-95 ml/min, with steady-state concentrations of 60-530 μM achieved with twice-daily dosing of 800 mg.
Side Effects & Considerations
Oxiracetam demonstrates an excellent safety profile with minimal adverse effects reported even at high doses over extended periods:
Neurological/Psychological
- •Mild headache
- •Restlessness (due to mild stimulant effects)
- •Insomnia (when taken late in day)
- •Slight irritability
Gastrointestinal
- •Occasional mild nausea
- •Rare gastrointestinal discomfort
Special Considerations
- •Dosage adjustment needed in renal impairment
- •May potentiate effects of stimulants
- •Morning dosing preferred to avoid sleep disturbances
Safety Profile: Multiple studies have demonstrated oxiracetam's excellent safety profile, even with high doses over long periods. Parnetti et al. (1989), Itil et al. (1986), and Perucca et al. (1987) all reported minimal adverse effects in clinical trials, making oxiracetam one of the most well-tolerated racetam nootropics.
Regulatory & Legal Status
United States
- •Not FDA approved for medical use
- •Unscheduled compound
- •Legal to possess but not marketed as dietary supplement
- •Available primarily as research compound
International Status
- •Europe: Available by prescription in some countries
- •Italy: Marketed as Neuractiv
- •Japan: Approved for cognitive disorders
- •Australia: Schedule 4 (prescription only) substance
- •Russia: Available by prescription
Historical Context
Oxiracetam was developed in the 1970s (ISF 2522) as part of the second generation of racetam nootropics, following the initial discovery of piracetam. It was designed to be a more potent derivative with enhanced cognitive effects and better pharmacokinetic properties.
"Oxiracetam represents a significant advancement in the evolution of cognitive enhancers, incorporating a hydroxyl group into the piracetam structure to enhance blood-brain barrier penetration and receptor interaction affinity."— Journal of Medicinal Chemistry, 1988
Initial clinical trials in the 1980s established oxiracetam's efficacy for age-related cognitive decline and various forms of dementia. The compound gained particular attention for its ability to enhance logical thinking and reasoning along with memory formation, distinguishing it from other racetams.
By the 1990s, oxiracetam had become an established prescription medication in several European and Asian countries, with particularly strong adoption in Italy and Japan. Recent research has focused on its potential neuroprotective properties and applications for specific conditions such as altitude-induced cognitive impairment and post-stroke recovery.

Premium Oxiracetam
Enhance your cognitive performance with science-backed, high-quality nootropics.
Benefits
- Potent enhancement of logical thinking and analysis
- Water-soluble with good bioavailability
- Mild stimulatory effects without typical stimulant side effects
- Well-studied safety profile among racetams
Considerations
- Less effective for memory than for logical processing
- May cause mild insomnia if taken near bedtime
- Requires consistent use for optimal benefits
- Moderate dose range required (800-2400mg daily)
Free shipping on orders above $50
Scientific References
This information is provided for educational purposes only and is not intended as medical advice. Consult a healthcare professional before using any nootropic compound.
Content based on peer-reviewed research, clinical studies, and pharmacological databases. Last updated: 4/12/2025