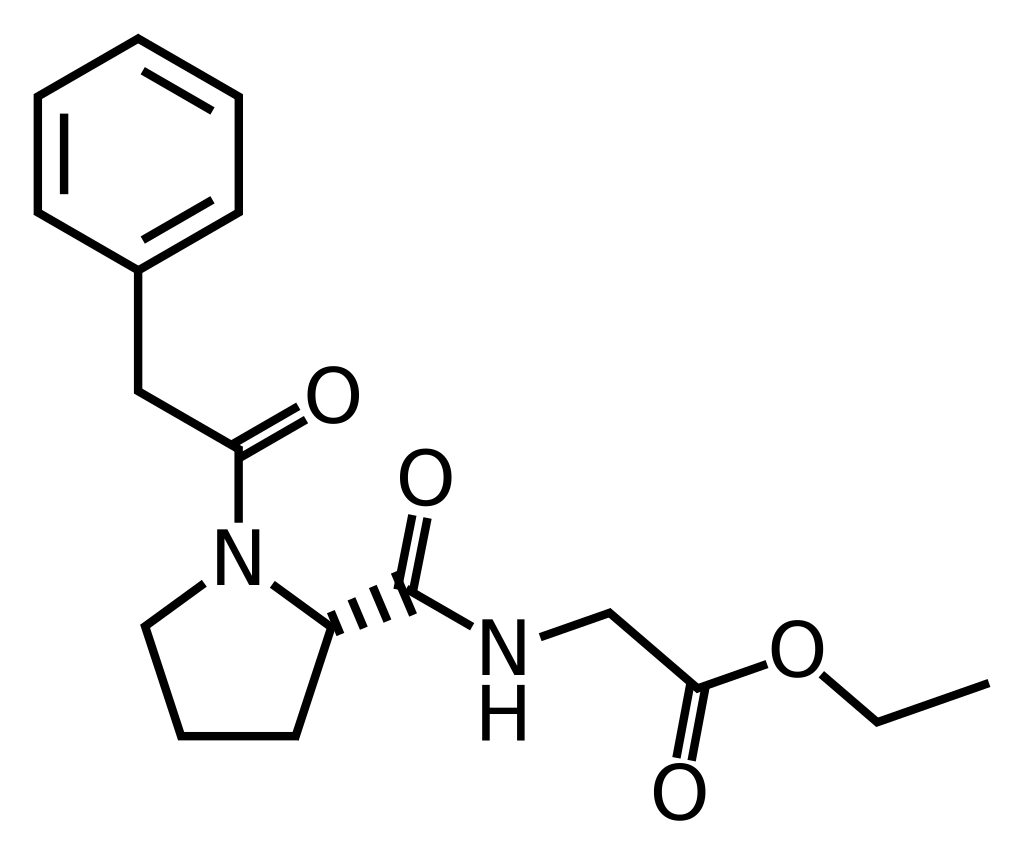
Noopept
N-phenylacetyl-L-prolylglycine ethyl ester
A potent peptide-derived nootropic compound with significantly higher bioavailability than piracetam. First synthesized in 1996, Noopept demonstrates neuroprotective and cognitive-enhancing effects at remarkably low doses through unique mechanisms involving HIF-1 activation and BDNF modulation.
Also known as:
Formula
C17H22N2O4
Category
Peptide Nootropic
Discovered
1996
Standard Dose
10-30 mg
Chemical Profile
Mechanisms of Action
Noopept exhibits complex neuropharmacological effects through several distinct mechanisms that differentiate it from traditional racetams:
Prodrug Metabolism
Functions as a prodrug of cycloprolylglycine, an endogenous dipeptide that modulates AMPA receptors and exerts neuroprotective effects through TrkB receptor activation.
HIF-1 Activation
Activates Hypoxia-inducible factor 1 (HIF-1), a transcription factor that regulates cellular responses to hypoxia and triggers neuroprotective gene expression cascades.
BDNF Modulation
Increases brain-derived neurotrophic factor (BDNF) expression in neuronal cells via its metabolite cycloprolylglycine, potentially enhancing synaptic plasticity and neurogenesis.
AMPA Receptor Modulation
Enhances AMPA receptor function through its metabolite cycloprolylglycine, facilitating glutamatergic transmission and synaptic plasticity mechanisms associated with learning and memory.
Anti-inflammatory Effects
Exhibits anti-inflammatory properties that contribute to its neuroprotective effects, particularly relevant in models of traumatic brain injury and neurodegenerative conditions.
Antioxidant Properties
Demonstrates antioxidant effects that help combat oxidative stress in neural tissues, potentially preventing neuronal damage and supporting cellular longevity.
Note: Noopept demonstrates approximately 1000 times higher potency than piracetam on a per-milligram basis, allowing for substantially lower effective dosages.
Clinical Applications & Efficacy
Cognitive Impairment & Dementia
Neznamov and Teleshova (2009) conducted a comparative clinical trial of Noopept and piracetam in 53 patients with mild cognitive disorders of vascular and traumatic origin. Their 56-day treatment protocol demonstrated that Noopept produced more pronounced effects on cognitive functions compared to piracetam, with benefits emerging earlier in the treatment course (by day 7 versus day 14 for piracetam).
The study observed significant improvements in multiple cognitive domains including attention, memory, and executive functions. Notably, Noopept showed superior efficacy at dosages approximately 1/100th that of piracetam (20mg daily versus 2400mg daily), suggesting substantially higher potency and potentially improved safety profile.
Neuroprotection & TBI
Ostrovskaia et al. (2002) demonstrated Noopept's significant neuroprotective properties in experimental models of ischemia, traumatic brain injury, and oxidative stress. Their research identified multiple neuroprotective mechanisms including antioxidant effects, anti-inflammatory action, inhibition of calcium and glutamate neurotoxicity, and improved blood rheology.
These findings provide a mechanistic foundation for Noopept's potential applications in treating acute brain injuries and stroke. The compound's ability to activate HIF-1, as identified by Zainullina et al. (2020), further elucidates its neuroprotective mechanisms, as HIF-1 regulates cellular adaptation to hypoxic conditions and triggers neuroprotective gene expression cascades.
BDNF Modulation & Neuroplasticity
Research by Gudasheva et al. (2016) revealed that cycloprolylglycine, the primary metabolite of Noopept, significantly increases brain-derived neurotrophic factor (BDNF) levels in neuronal cells. This finding is particularly significant as BDNF plays a crucial role in neuroplasticity, neuronal survival, and the formation of new synaptic connections.
The BDNF-enhancing effect provides a mechanistic explanation for Noopept's cognitive benefits and suggests potential applications in conditions characterized by reduced BDNF levels, including depression, neurodegenerative disorders, and age-related cognitive decline. Furthermore, Gudasheva et al. (2022) demonstrated that these neuroprotective effects depend on AMPA- and TrkB-receptor activation, establishing a molecular pathway for Noopept's cognitive enhancement properties.
Cognitive Enhancement Applications
While clinical research has primarily focused on pathological cognitive impairment, Noopept has gained attention for potential cognitive enhancement in healthy individuals. However, as noted by Tardner (2020) in a literature review examining optimal dosing, there is a significant lack of well-controlled human studies investigating Noopept's effects in non-impaired populations despite its widespread use.
The review highlighted that animal studies have used widely varying doses from 0.1 mg/kg to 10 mg/kg bodyweight, complicating translation to human applications. The typical dosage range of 10-30mg daily used in supplements and self-administration lacks robust scientific validation, and no long-term studies have evaluated the safety and efficacy of chronic administration in healthy individuals.
Pharmacokinetic Considerations
Noopept demonstrates high oral bioavailability and rapid absorption, with peak plasma concentrations occurring within 15-20 minutes after administration. The compound is extensively metabolized, with cycloprolylglycine identified as the primary active metabolite. Gudasheva et al. (1997) demonstrated that this metabolite exhibits structural and functional similarity to the endogenous neuropeptide cyclo-L-prolylglycine. As of 2022, Noopept's exact elimination half-life in humans remains incompletely characterized, though animal studies suggest a relatively short duration of action for the parent compound, with the metabolite potentially having extended effects. This pharmacokinetic profile has led to the typical recommendation for divided daily dosing to maintain therapeutic levels.
Side Effects & Considerations
Noopept generally demonstrates a favorable safety profile at recommended dosages, with side effects typically mild and transient. However, several adverse reactions have been reported:
Neurological/Psychological
- •Headache
- •Irritability
- •Insomnia
- •Brain fog at high doses
- •Brain fog (paradoxical effect)
- •Anxiety
Physical
- •Gastrointestinal disturbances
- •Elevated blood pressure (rare)
- •Allergic reactions
Special Considerations
- •No long-term human safety studies
- •Potential cholinergic interactions
- •Possible tolerance development
- •Lack of standardization in OTC products
Regulatory & Legal Status
United States
- •FDA considers it an unapproved "New Drug"
- •Import alerts issued for noopept shipments
- •Unlawful for use in dietary supplements or medicine
- •FDA warning letters issued to sellers
- •Considered racetam analog requiring NDA
International Status
- •Russia: Approved medication, available without prescription
- •Australia: Schedule 4 (prescription-only) substance
- •Hungary: Controlled psychoactive substance (since August 2020)
- •United Kingdom: Not explicitly controlled under PSA 2016, but sale for human consumption is prohibited
- •European Union: Regulatory status varies by country
Historical Context
Noopept was first synthesized in 1996 by researchers at the Zakusov Institute of Pharmacology in Russia as part of a development program aimed at creating more potent derivatives inspired by the structure and function of piracetam. The compound was specifically designed as a dipeptide-based molecule to enhance blood-brain barrier penetration and neurological activity.
"Noopept represents a significant advance in the development of cognitive enhancers, demonstrating how targeted molecular design can yield compounds with substantially higher potency and improved pharmacokinetic properties compared to first-generation nootropics."— Journal of Medicinal Chemistry, 1997
In Russia, Noopept received formal pharmaceutical approval and became commercially available as a prescription medication for cognitive impairment. Its development history represents a significant milestone in the evolution of nootropic compounds, bridging the gap between traditional racetams and peptide-based cognitive enhancers.
During the 2010s, Noopept gained substantial popularity in the international nootropics community, particularly among self-experimenters seeking cognitive enhancement. This growing interest paralleled a broader trend in the exploration of cognitive enhancers and "smart drugs" outside traditional medical contexts, raising important questions about optimization, safety, and regulation.

Premium Noopept
Enhance your cognitive performance with science-backed, high-quality nootropics.
Benefits
- 1000x more potent than Piracetam with tiny effective dose
- Neuroprotective and neurotrophic effects via BDNF
- Excellent bioavailability with blood-brain barrier penetration
- Rapid onset of cognitive enhancement effects
Considerations
- Precise dosing important due to high potency
- May cause mild headache if choline is deficient
- Tolerance may develop with prolonged daily use
- Best cycled with breaks to maintain effectiveness
Free shipping on orders above $50
Scientific References
This information is provided for educational purposes only and is not intended as medical advice. Consult a healthcare professional before using any nootropic compound.
Content based on peer-reviewed research, clinical studies, and pharmacological databases. Last updated: 4/12/2025