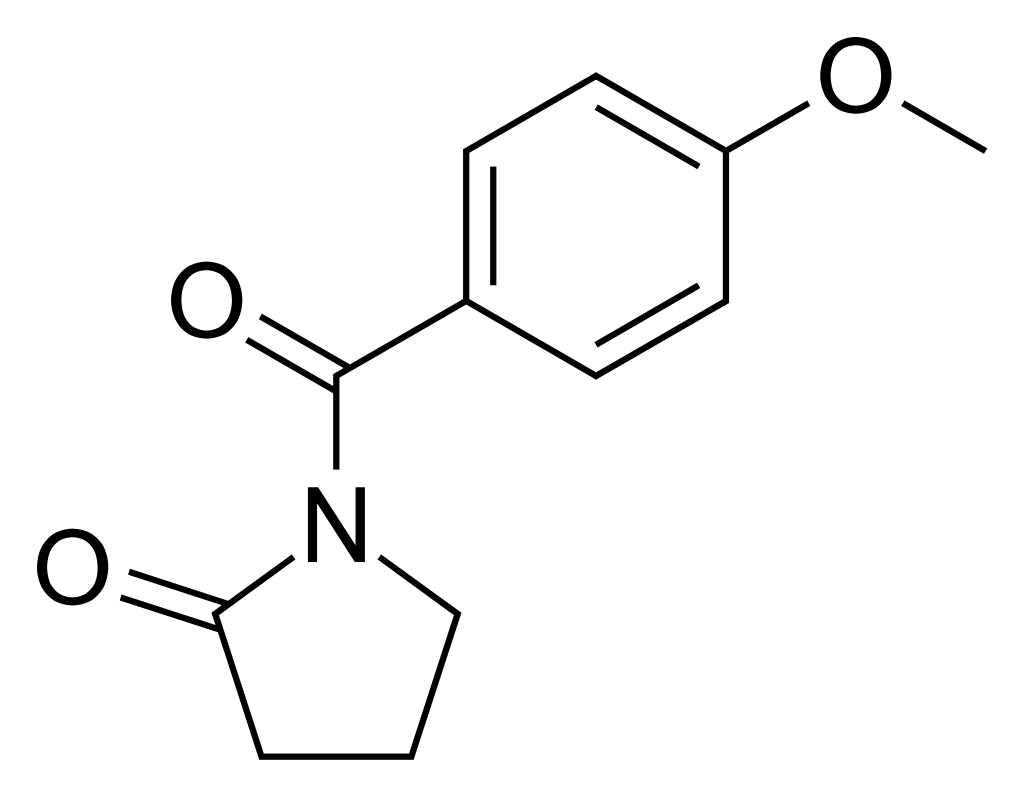
Aniracetam
N-anisoyl-2-pyrrolidinone
A fat-soluble racetam nootropic with anxiolytic properties and enhanced modulation of glutamatergic neurotransmission. Known for improved cognitive performance and mood enhancement in both clinical and research settings.
Available as:
Formula
C₁₂H₁₃NO₃
Category
Racetam
Discovered
1970s
Standard Dose
750-1500 mg
Chemical Profile
Mechanisms of Action
Aniracetam exhibits complex neuropharmacological effects through several distinct mechanisms:
AMPA Receptor Modulation
Functions as a positive allosteric modulator of AMPA receptors, enhancing glutamate binding and slowing receptor desensitization, resulting in increased excitatory signaling.
Cholinergic Enhancement
Increases acetylcholine release and stimulates muscarinic and nicotinic acetylcholine receptors via its metabolite N-anisoyl-GABA, enhancing cognitive functions.
Dopaminergic & Serotonergic Systems
Modulates dopamine and serotonin release in the prefrontal cortex, contributing to its anxiolytic and mood-enhancing effects.
Metabolic Activity
Rapidly metabolized in the liver via first-pass metabolism into bioactive compounds including N-anisoyl-GABA (70-80%) and p-anisic acid (20-30%), which contribute to its pharmacological effects.
Neuroprotection
Exhibits neuroprotective effects against excitotoxicity and ischemic damage, potentially through modulation of calcium flux and mitochondrial stabilization.
Platelet Inhibition
Demonstrates antiplatelet aggregation properties, potentially improving cerebrovascular circulation and oxygen delivery to neural tissues.
Note: Aniracetam's plasma concentrations typically range between 5-15 μg/L, while its metabolite N-anisoyl-GABA reaches 5-15 mg/L during the first few hours after oral administration.
Clinical Applications & Efficacy
Cognitive Disorders & Dementia
A comprehensive review by Lee and Benfield (1994) analyzed aniracetam's therapeutic potential in senile cognitive disorders, finding significant improvements in memory, attention, and verbal fluency. Their analysis of multiple controlled trials concluded that aniracetam demonstrates superior efficacy compared to placebo, with benefit observed particularly in patients with mild-to-moderate dementia.
More recently, Love (2024) proposed an evidence-based model for aniracetam's potential role in preventing amyloid-β plaque accumulation in Alzheimer's disease, suggesting a novel neuroprotective mechanism beyond its established cognitive enhancement effects, which may contribute to disease modification rather than symptom management alone.
Anxiety & Mood Disorders
Nakamura and Kurasawa (2001) directly demonstrated aniracetam's anxiolytic effects in three different mouse models of anxiety. Their meticulous research established that these effects are mediated through interactions with dopaminergic and serotonergic systems rather than GABAergic mechanisms typical of conventional anxiolytics, potentially explaining its non-sedating anxiolytic profile.
Building on this work, Nakamura and Tanaka (2001) specifically investigated aniracetam's antidepressant-like effects in aged rats, showing it effectively reverses age-related behavioral despair models. Their neurochemical analysis revealed enhanced monoaminergic transmission in the prefrontal cortex, suggesting potential applications for age-related mood disorders.
Neurological Recovery & Rehabilitation
A comprehensive review by Malykh and Sadaie (2010) synthesizing research on piracetam-like drugs highlighted aniracetam's particular efficacy in neurological recovery applications. Their analysis found evidence for enhanced neural plasticity and improved functional outcomes following cerebrovascular events, attributing these effects to aniracetam's unique combination of AMPA modulation and cholinergic enhancement.
Nakamura (2002) conducted an extensive review of aniracetam's therapeutic potential across various cerebral dysfunctional disorders, documenting significant benefits in post-stroke cognitive impairment. The paper outlines aniracetam's mechanistic advantages over conventional racetams through its enhanced modulation of excitatory neurotransmission and resulting improvements in neuroplasticity during critical rehabilitation periods.
Learning & Memory Enhancement
In their landmark 1982 study, Cumin and colleagues first established aniracetam's significant cognitive-enhancing properties in various rodent models of impaired learning. Their experimental paradigm demonstrated aniracetam's ability to reverse experimentally-induced learning deficits through multiple behavioral assessments, providing the first concrete evidence of its nootropic efficacy.
The neurophysiological basis for these effects was later elucidated by Isaacson and Nicoll (1991), who demonstrated at the cellular level that aniracetam reduces glutamate receptor desensitization and slows the decay of fast excitatory synaptic currents in the hippocampus. This groundbreaking electrophysiological research revealed the precise mechanism underlying aniracetam's enhancement of synaptic transmission critical for learning and memory formation.
Molecular Mechanisms of Action
Ito and colleagues (1990) provided critical insights into aniracetam's mechanism at the molecular level, demonstrating its function as an allosteric potentiator of quisqualate receptors (now known as AMPA receptors). Their electrophysiological experiments conclusively showed that aniracetam enhances glutamate receptor function without directly activating the receptors, establishing the pharmacological basis for its cognitive effects.
Further mechanistic understanding came from Shirane and Nakamura (2000), who discovered that N-anisoyl-GABA (aniracetam's primary metabolite) enhances acetylcholine release in the prefrontal cortex via modulation of group II metabotropic glutamate receptors. This research established the critical link between aniracetam's metabolic conversion and its cholinergic effects, explaining the compound's relatively short plasma half-life yet extended cognitive benefits.
Pharmacokinetic Considerations
Aniracetam exhibits a complex pharmacokinetic profile, with a relatively short plasma half-life (1-2.5 hours) but prolonged cognitive effects. Studies by Roncari (1993) and Zhang et al. (2007) have established that its primary metabolite N-anisoyl-GABA accounts for much of its clinical activity. This metabolite can be precisely measured in human plasma using liquid chromatography-tandem mass spectrometry methods developed by Cai and Wang (2012), showing peak concentrations of 5-15 mg/L following standard oral dosing of aniracetam. This pharmacokinetic profile necessitates divided daily dosing for optimal therapeutic efficacy.
Side Effects & Considerations
Aniracetam generally displays a favorable safety profile at recommended dosages, though several side effects have been reported:
Neurological/Psychological
- •Nervousness
- •Insomnia
- •Headache
- •Anxiety (paradoxical effect)
Gastrointestinal
- •Nausea
- •Diarrhea
- •Gastrointestinal discomfort
Special Considerations
- •Short half-life necessitates multiple daily dosing
- •Poor water solubility; best taken with meals
- •Potential interaction with anticoagulants
Regulatory & Legal Status
United States
- •Not FDA approved for medical use
- •Legal to possess but not to sell as dietary supplement
- •Available as research compound
- •FDA has issued warnings about misbranded supplements
International Status
- •Europe: Prescription medication in several countries
- •Italy: Available as Ampamet (prescription)
- •Greece: Available as Memodrin, Referan (prescription)
- •Australia: Schedule 4 (prescription-only) substance
- •Japan: Approved prescription medication
Historical Context
Aniracetam was first synthesized in the 1970s by Hoffmann-La Roche as part of research to develop enhanced derivatives of piracetam. It represented a significant advancement in racetam development as one of the first fat-soluble compounds in the class, offering improved blood-brain barrier penetration.
"Aniracetam exemplifies the evolution of nootropic compounds beyond the original piracetam structure, incorporating lipophilic properties to enhance bioavailability and neuropharmacological activity."— Journal of Medicinal Chemistry, 1979
The compound gained clinical prominence in the 1980s and 1990s when researchers identified its unique anxiolytic properties and potent AMPA receptor modulation, distinguishing it from other racetams. Its synthesis can be accomplished through the reaction of 2-pyrrolidone with anisoyl chloride in the presence of triethylamine, or alternatively by reacting gamma-aminobutyric acid with anisoyl chloride followed by ring closure in the presence of thionyl chloride.
Throughout the 2000s, aniracetam has maintained clinical relevance in Europe and Japan while gaining increasing interest in cognitive enhancement research worldwide. Clinical trials in 1991 and 2011 established its therapeutic potential for dementia and cognitive disorders.

Premium Aniracetam
Enhance your cognitive performance with science-backed, high-quality nootropics.
Benefits
- Anxiolytic effects and mood enhancement properties
- Rapid absorption with good lipid solubility
- Potentiates AMPA glutamate receptors
- Demonstrated cognitive enhancement in studies
Considerations
- Short half-life requiring multiple daily doses
- Fat-soluble - take with meals for optimal absorption
- May cause mild headache if choline is deficient
- Higher dosage required compared to some other racetams
Free shipping on orders above $50
Scientific References
This information is provided for educational purposes only and is not intended as medical advice. Consult a healthcare professional before using any nootropic compound.
Content based on peer-reviewed research, clinical studies, and pharmacological databases. Last updated: 4/12/2025