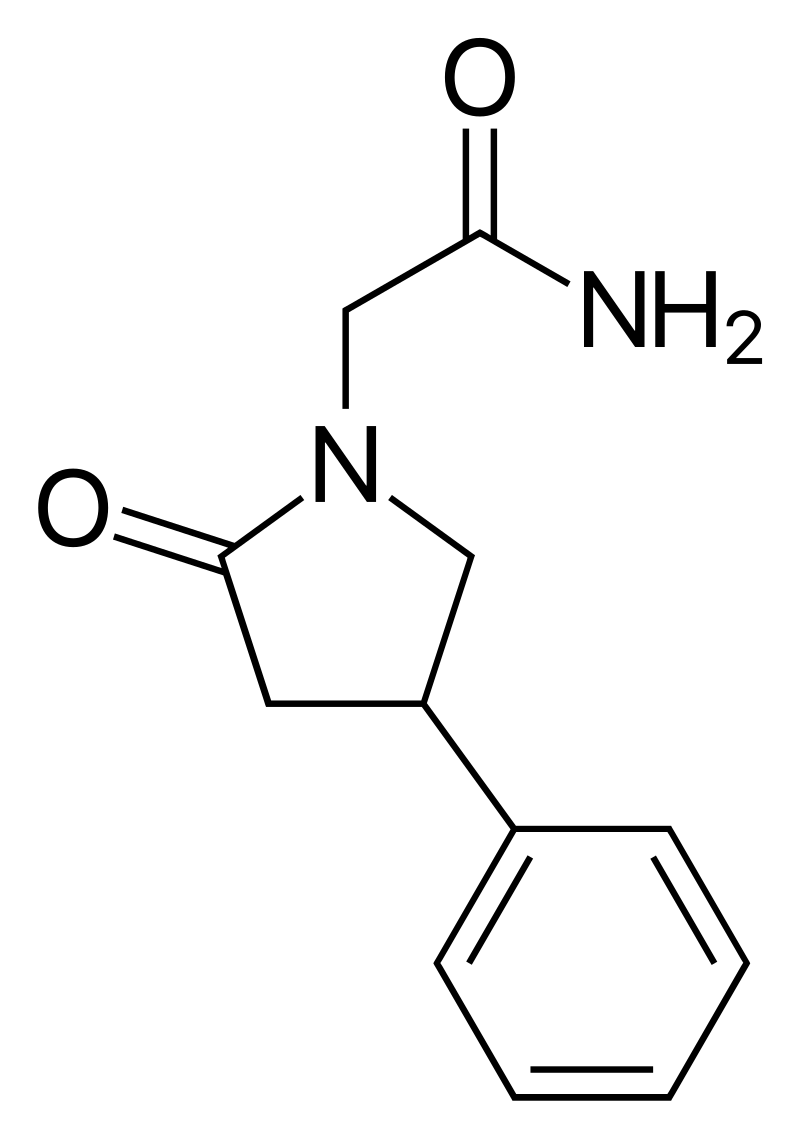
Phenylpiracetam
(RS)-2-(2-oxo-4-phenylpyrrolidin-1-yl)acetamide
A high-potency racetam derivative with powerful stimulatory effects and dopamine reuptake inhibition properties. Known for significantly enhancing physical performance, cold tolerance, and cognitive function under stressful conditions.
Available as:
Formula
C₁₂H₁₄N₂O₂
Category
Racetam
Half-Life
3-5 hours
Standard Dose
100-200 mg
Chemical Profile
Mechanisms of Action
Phenylpiracetam exhibits multiple neuropharmacological effects with distinct stereoselective properties between its (R)- and (S)-enantiomers:
Selective Dopamine Reuptake Inhibition
(R)-Phenylpiracetam acts as an atypical dopamine transporter (DAT) inhibitor with IC₅₀ values of 4.82 μM for DAT binding and 14.5 μM for dopamine uptake inhibition in human recombinant DAT (Sommer et al., 2014). This mechanism significantly contributes to its stimulatory and motivational effects.
Norepinephrine Transporter Modulation
(R)-Phenylpiracetam inhibits the norepinephrine transporter (NET) with an IC₅₀ of approximately 182 μM, functioning as a dual norepinephrine–dopamine reuptake inhibitor (NDRI), albeit with 11-fold lower affinity for NET than for DAT (Sommer et al., 2014; Zvejniece et al., 2020).
Nicotinic Acetylcholine Receptor Binding
Binds to α4β2 nicotinic acetylcholine receptors in the cerebral cortex with an IC₅₀ of 5.86 μM, modulating cholinergic neurotransmission as demonstrated by Voronina (2023) and Zhao et al. (2001) in studies on rat cortical neurons.
AMPA Receptor Potentiation
Like other racetams, phenylpiracetam appears to potentiate AMPA receptors, facilitating glutamatergic neurotransmission and enhancing synaptic plasticity, which may contribute to memory formation and learning processes.
Enantiomer-Specific Actions
The (R)-enantiomer is substantially more potent in stimulating locomotor activity than the (S)-enantiomer. While (S)-phenylpiracetam also shows DAT inhibition (with lower potency), it does not significantly stimulate locomotor activity, suggesting complex stereoselective mechanisms.
Neuroprotective Properties
Demonstrates protective effects against hypoxia and cerebral ischemia, improving cerebral blood flow and restoring neurological function more effectively than piracetam in animal models of gravitational cerebral ischemia.
Note: Phenylpiracetam is the only racetam officially banned by the World Anti-Doping Agency (WADA) due to its pronounced stimulatory effects and demonstrated enhancement of physical performance (Docherty, 2008; Smith et al., 2020). This unique regulatory status reflects its substantially different pharmacological profile compared to other members of the racetam family.
Clinical Applications & Efficacy
Cerebrovascular Disorders & Cognitive Recovery
Clinical studies at the Russian Center of Vegetative Pathology have demonstrated phenylpiracetam's efficacy in improving regional blood flow in ischemic regions of the brain. Research by Savchenko et al. (2005) found significant improvements in patients with lesions of cerebral blood pathways, providing evidence for phenylpiracetam's application in post-stroke cognitive rehabilitation.
In a study conducted by Gustov et al. (2006), phenylpiracetam was shown to reduce neurological deficits following vascular encephalopathy, with patients demonstrating improved memory, attention, and cognitive processing compared to control groups.
Asthenia & Fatigue Reduction
Kalinskiĭ and Nazarov (2007) investigated phenylpiracetam's effects on asthenic syndrome and autonomic disturbances following mild cranial brain trauma. Their findings revealed significant improvements in energy levels, reduced fatigue, and normalized autonomic function in the treatment group compared to controls.
Research documented by Voronina (2023) demonstrated phenylpiracetam's ability to increase physical and mental work capacity under conditions of extreme stress and fatigue. It was specifically developed at the Institute of Biomedical Problems as a new generation psychostimulant to enhance the performance of astronauts during various stages of space flight.
Depression & Anxiety Disorders
Clinical trials at the Serbsky State Scientific Center for Social and Forensic Psychiatry evaluated phenylpiracetam's effects on depressive and anxiety disorders. The compound demonstrated a psycho-activating effect and successfully reduced symptoms of depression and anxiety in patients with various neuropsychiatric conditions.
Additionally, research by Akhapkina & Akhapkin (2013) showed phenylpiracetam has neuroleptic activity and improves sleep quality while simultaneously increasing daytime alertness and energy, making it unique among stimulants which typically disrupt sleep architecture. This dual action may contribute to its efficacy in treating depression with comorbid fatigue and sleep disturbances.
Epilepsy (Adjunct Therapy)
Lybzikova et al. (2008) investigated phenylpiracetam's efficacy as an add-on treatment in epilepsy. The study found that phenylpiracetam exhibited significant anticonvulsant properties when used as an adjunct to standard antiepileptic medications, potentially through modulating neuronal excitability and enhancing GABA-ergic inhibitory neurotransmission.
The anticonvulsant effects were particularly pronounced in patients with epilepsy complicated by cognitive impairment, where phenylpiracetam provided dual benefits of seizure reduction and improved cognitive function, making it a valuable adjunct in these difficult-to-treat cases.
Physical Performance Enhancement
In controlled animal studies documented by Sommer et al. (2014), phenylpiracetam demonstrated remarkable effects on motivation and work capacity. Researchers found that the (R)-enantiomer of phenylpiracetam (MRZ-9547) significantly increased progressive ratio responding in rats, indicating enhanced motivation to expend effort for rewards.
These findings align with human studies by Malykh and Sadaie (2010), suggesting phenylpiracetam may significantly enhance not only physical endurance but also motivation and willingness to engage in effortful activities. This unique combination of effects led to phenylpiracetam being the only racetam explicitly banned by WADA for use in competitive sports (Docherty, 2008).
Pharmacokinetic Considerations
Phenylpiracetam exhibits favorable pharmacokinetic properties with nearly 100% oral bioavailability and rapid absorption occurring within 1 hour after administration. According to studies by Antonova et al. (2003), it reaches peak effects between 1-3 hours post-ingestion and has a relatively short half-life of 3-5 hours in humans. Unlike many pharmaceuticals, phenylpiracetam appears to undergo minimal metabolism, with approximately 40% excreted unchanged in urine and the remaining 60% eliminated via bile and sweat. These properties contribute to its rapid onset, relatively brief but potent effects, and low risk of accumulation with repeated dosing.
Side Effects & Considerations
Phenylpiracetam has a relatively favorable safety profile but may cause stimulant-like side effects at higher dosages:
Neurological/Psychological
- •Insomnia or sleep disturbances (if taken late in day)
- •Psychomotor agitation
- •Increased anxiety or irritability
- •Headache
Cardiovascular
- •Increased blood pressure
- •Flushing or feeling of warmth
- •Increased heart rate
Special Considerations
- •Anorexic effect with extended use
- •Potential for tolerance development
- •May interact with other stimulants
Safety Profile: No serious overdoses have been reported with phenylpiracetam, and clinical experience suggests a wide safety margin. Unlike conventional stimulants, phenylpiracetam does not typically produce severe cardiovascular effects or significant withdrawal syndromes. However, as a dopamine reuptake inhibitor, caution is advised when combining with other substances that affect dopaminergic neurotransmission.
Regulatory & Legal Status
United States
- •Not FDA approved for medical use
- •Unscheduled by the DEA
- •Use in dietary supplements, food, or medicine is unlawful
- •Available primarily as a research compound
International Status
- •Russia: Approved prescription medication since 2003
- •Eastern Europe: Available by prescription in several countries
- •Australia: Schedule 4 (prescription only) substance
- •World Anti-Doping Agency: Prohibited substance in competitive sports
Anti-Doping Status: Phenylpiracetam is the only racetam compound specifically prohibited by the World Anti-Doping Agency (WADA). It is listed as a non-specified stimulant prohibited in-competition under Section S6 of the WADA Prohibited List (Smith et al., 2020; Docherty, 2008). This prohibition reflects its significant stimulant properties and demonstrated ability to enhance physical performance.
Historical Context
Phenylpiracetam was developed in 1983 at the Russian Academy of Sciences Institute of Biomedical Problems under the leadership of psychopharmacologist Valentina Ivanovna Akhapkina. It was first described in scientific literature by Bobkov et al. (1983) and specifically created for Soviet cosmonauts to combat the extreme physiological and psychological stresses of prolonged space missions (Voronina, 2023; Veinberg et al., 2015).
"The drug acts as the equalizer of the whole organism, 'tidying it up,' completely excluding impulsiveness and irritability inevitable in the stressful conditions of space flight."— Aleksandr Serebrov, Pilot-Cosmonaut, after 197 days aboard Mir space station
Following its successful use in the Soviet/Russian space program, phenylpiracetam was included in the Soyuz spacecraft's standard emergency medical kit. It became commercially available as a pharmaceutical in Russia in the early 2000s, receiving official approval for medical use in 2003.
In recent years, research has advanced understanding of phenylpiracetam's mechanisms, particularly the 2014 discovery by Sommer et al. of (R)-phenylpiracetam's action as a selective atypical dopamine reuptake inhibitor. More recently, development of (R)-phenylpiracetam (code-named MRZ-9547) has been explored in the West as a potential treatment for fatigue related to Parkinson's disease and other conditions (Stutz et al., 2019).
The compound's notable stimulant properties led to its inclusion on the World Anti-Doping Agency's prohibited substances list, where it remains the only racetam explicitly banned in competitive sports, highlighting its pharmacological uniqueness within the racetam family.

Premium Phenylpiracetam
Enhance your cognitive performance with science-backed, high-quality nootropics.
Benefits
- 30-60x more potent than Piracetam with psychostimulatory effects
- Enhanced physical performance and cold resistance
- Improved focus, concentration and motivation
- Rapid onset of action within 30-60 minutes
Considerations
- Banned by WADA for competitive athletes
- Tolerance develops quickly with daily use
- Best used occasionally or cyclically (1-2x per week)
- May increase anxiety in sensitive individuals
Free shipping on orders above $50
Scientific References
This information is provided for educational purposes only and is not intended as medical advice. Consult a healthcare professional before using any nootropic compound.
- Bobkov Iu, et al. (1983). Pharmacological characteristics of a new phenyl analog of piracetam--4-phenylpiracetam. Biull Eksp Biol Med, 95(4):50-53.
- Docherty JR (2008). Pharmacology of stimulants prohibited by the World Anti-Doping Agency (WADA). Br J Pharmacol, 154(3):606-622.
- Gromova OA, Torshin IY (2024). Pharmacological effects of fonturacetam (Actitropil) and prospects for its clinical use. S.S. Korsakov Journal of Neurology and Psychiatry, 124(8):21-31.
- Malykh AG, Sadaie MR (2010). Piracetam and piracetam-like drugs: from basic science to novel clinical applications to CNS disorders. Drugs, 70(3):287-312.
- Sommer S, et al. (2014). The dopamine reuptake inhibitor MRZ-9547 increases progressive ratio responding in rats. Int J Neuropsychopharmacol, 17(12):2045-2056.
- Veinberg G, et al. (2015). Stereochemistry of phenylpiracetam and its methyl derivative: improvement of the pharmacological profile. Chemistry of Heterocyclic Compounds, 51(7):601-606.
- Voronina TA (2023). Cognitive Impairment and Nootropic Drugs: Mechanism of Action and Spectrum of Effects. Neurochemical Journal, 17(2):180-188.
- Zvejniece L, et al. (2020). Neuroprotective and anti-inflammatory activity of DAT inhibitor R-phenylpiracetam in experimental models of inflammation in male mice. Inflammopharmacology, 28(5):1283-1292.
Content based on peer-reviewed research, clinical studies, and pharmacological databases. Last updated: 4/12/2025