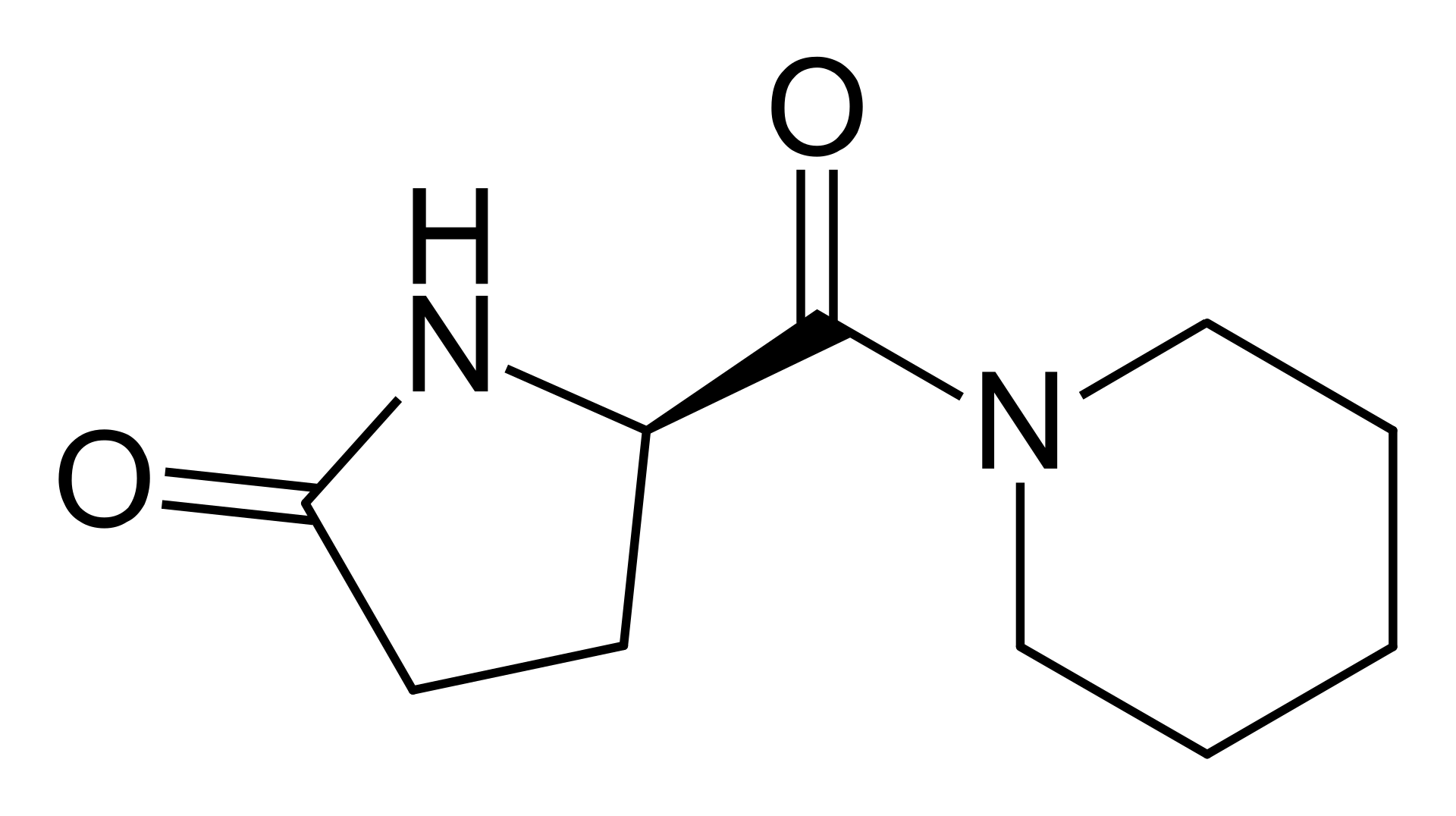
Fasoracetam
(NS-105, AEVI-001, NFC-1)
Fasoracetam is an experimental racetam that acts as an mGluR modulator and failed late-stage trials for vascular dementia and ADHD, but continues to be explored in rare genetic disorders and as a research cognitive enhancer.
Also known as:
Formula
C₁₀H₁₆N₂O₂
Category
Racetam / mGluR Modulator
Half-Life
4–6.5 hours
Bioavailability
79–97% (rodents)
Chemical Profile
Mechanism of Action
Fasoracetam is believed to modulate metabotropic glutamate receptors (mGluR groups 1, 2, and 3), increasing acetylcholine release and normalizing disrupted glutamatergic signaling. These properties are unique among studied racetams.
Preclinical & Clinical Evidence
Cognition Improvement in Animal Models
Fasoracetam demonstrated improvement of cognitive function, including memory and learning, in multiple rodent studies.
ADHD Linked to mGluR Mutations: Nature Communications 2018
In adolescents with ADHD and gene network variants that disrupt mGluR neurotransmitter signaling, Fasoracetam demonstrated some efficacy, but only in this rare subgroup.
Systematic Review of New ADHD Compounds
Broader reviews of RCTs, including those using Fasoracetam for ADHD, conclude that efficacy outside rare gene variant subgroups is limited or disappointing.
Safety & Side Effects
No significant safety signals emerged in published clinical studies, but the number/scale of trials is very limited, and rare effects may be undetected. Human use is not authorized outside research.
Regulatory & Legal Status
US/EU/International
- Not FDA- or EMA-approved for any indication
- Schedule 4 (Rx-only) in Australia
- Research chemical, not authorized for regular human/clinical use
Note: Not for human use or sale as a supplement.
History & Development
Developed by Nippon Shinyaku in the late 1980s, Fasoracetam underwent phase 3 trials for vascular dementia and later, for rare forms of ADHD. Development has since been largely discontinued except for rare genetic indications.

Premium Fasoracetam
Enhance your cognitive performance with science-backed, high-quality nootropics.
Benefits
- Unique mGluR modulator racetam
- Procognitive effect in animal data
- First-in-class compound for rare gene variant ADHD/DiGeorge research
Considerations
- No strong efficacy in general ADHD/human cognition
- Not FDA/EMA approved; Rx-only AU
- Not for human use
Free shipping on orders above $50
Scientific References
- Malykh, A.G., & Sadaie, M.R. “Piracetam and piracetam-like drugs: from basic science to novel clinical applications to CNS disorders.” Drugs, vol. 70, no. 3, 2010, pp. 287–312. https://doi.org/10.2165/11319230-000000000-00000.
- Elia, J., et al. “Fasoracetam in adolescents with ADHD and glutamatergic gene network variants disrupting mGluR neurotransmitter signaling.” Nature Communications, vol. 9, no. 1, 2018, p. 4. https://doi.org/10.1038/s41467-017-02244-2.
- Nageye, F., & Cortese, S. “Beyond stimulants: a systematic review of randomised controlled trials assessing novel compounds for ADHD.” Expert Review of Neurotherapeutics, vol. 19, no. 7, 2019, pp. 707–717. https://doi.org/10.1080/14737175.2019.1628640.
- "Fasoracetam - Avalo Therapeutics." AdisInsight, Springer Nature, updated 2021. https://adisinsight.springer.com/drugs/800024060.
- "Fasoracetam." PubChem Compound Summary, National Center for Biotechnology Information, CID 198695, 2024. https://pubchem.ncbi.nlm.nih.gov/compound/198695.
Information is for scientific and educational use only. Not for human or clinical application. Last updated: 4/12/2025.