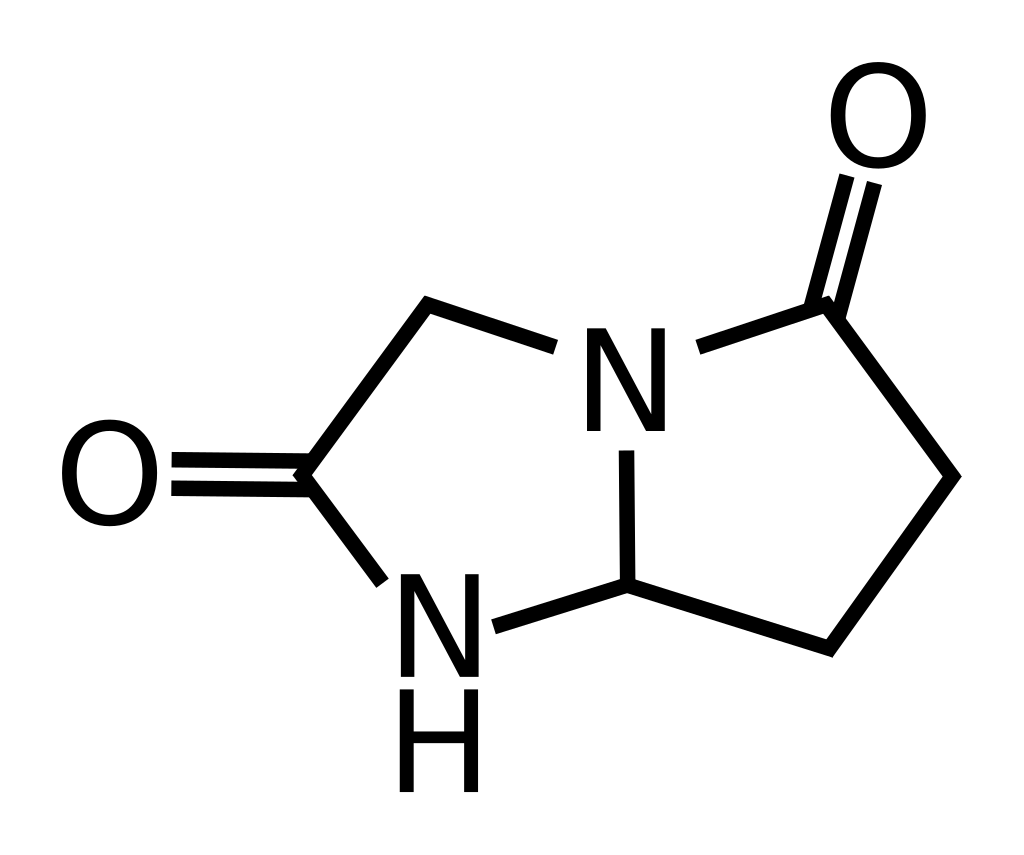
Dimiracetam
(RS)-3,6,7,7a-Tetrahydro-1H-pyrrolo[1,2-a]imidazole-2,5-dione
Dimiracetam is an experimental racetam nootropic displaying comparable or greater potency than piracetam in preclinical cognitive models, with unique pyrroloimidazolone structure. Several derivatives are also under investigation for neuropathic pain.
Formula
C₆H₈N₂O₂
Category
Racetam / Pyrroloimidazolone
Molar Mass
140.14 g/mol
Legal Status
Rx-only (AU), research elsewhere
Chemical Profile
Mechanism of Action
As with other racetams, Dimiracetam’s precise mechanism is undefined but is presumed to modulate central neurotransmission and synaptic plasticity, potentially acting via AMPA or other glutamatergic receptor systems. Some derivatives may have additional effects relevant to neuropathic pain.
Clinical & Preclinical Evidence
Cognitive Enhancement in Preclinical Models
Dimiracetam and analogues showed notable activity in rodent models (Morris water maze, passive avoidance, radial maze), often with greater potency than piracetam. The originally published series includes derivatives as well as the parent compound.
Pinza M, Farina C, Cerri A, et al. J Med Chem. 1993 Dec;36(26):4214-20.
Dimiracetam Derivatives: Neuropathic Pain Activity
Recent analogues display potent and stereoselective efficacy in animal models of neuropathic pain, including chronic constriction and spinal nerve injury paradigms.
Farina C, Gagliardi S, Ghelardini C, et al. Bioorg Med Chem. 2008 Mar;16(6):3224-32.
Safety & Side Effects
No specific side effect or toxicity profile has been published for dimiracetam in either animal or clinical studies; assumed low in acute animal testing, but human safety is not established.
Regulatory & Legal Status
Australia
- Schedule 4 (Rx Only) under the Poisons Standard
- Not approved for general medical use
US/EU/International
- Not FDA or EMA approved; research only
- Legal status: unscheduled in most regions
Note: Dimiracetam is not approved for human use in any country except as a potential prescription/research compound in Australia.
Development History
Dimiracetam was developed in the early 1990s as a novel racetam with an imidazolone structure distinct from piracetam. Its analogues are still studied for potential in neuropathic pain treatment, but neither the parent drug nor derivatives are available as pharmaceuticals.

Premium Dimiracetam
Enhance your cognitive performance with science-backed, high-quality nootropics.
Benefits
- Unique pyrroloimidazolone racetam structure
- Potent in preclinical cognition tests
- Some derivatives highly active in pain models
Considerations
- No FDA or EU regulatory approval
- Research chemical only; use restrictions apply
- No human safety data
Free shipping on orders above $50
Scientific References
- Pinza M, Farina C, Cerri A, Pfeiffer U, Riccaboni MT, Banfi S, et al. Synthesis and pharmacological activity of a series of dihydro-1H-pyrrolo[1,2-a]imidazole-2,5(3H,6H)-diones, a novel class of potent cognition enhancers. J Med Chem. 1993 Dec;36(26):4214-20.
- Farina C, Gagliardi S, Ghelardini C, Martinelli M, Norcini M, Parini C, et al. Synthesis and biological evaluation of novel dimiracetam derivatives useful for the treatment of neuropathic pain. Bioorg Med Chem. 2008;16(6):3224–32.
- Poisons Standard February 2020. Australia S4 listing.
This summary is for research and educational purposes only. Not medical advice. Last updated: 4/12/2025.